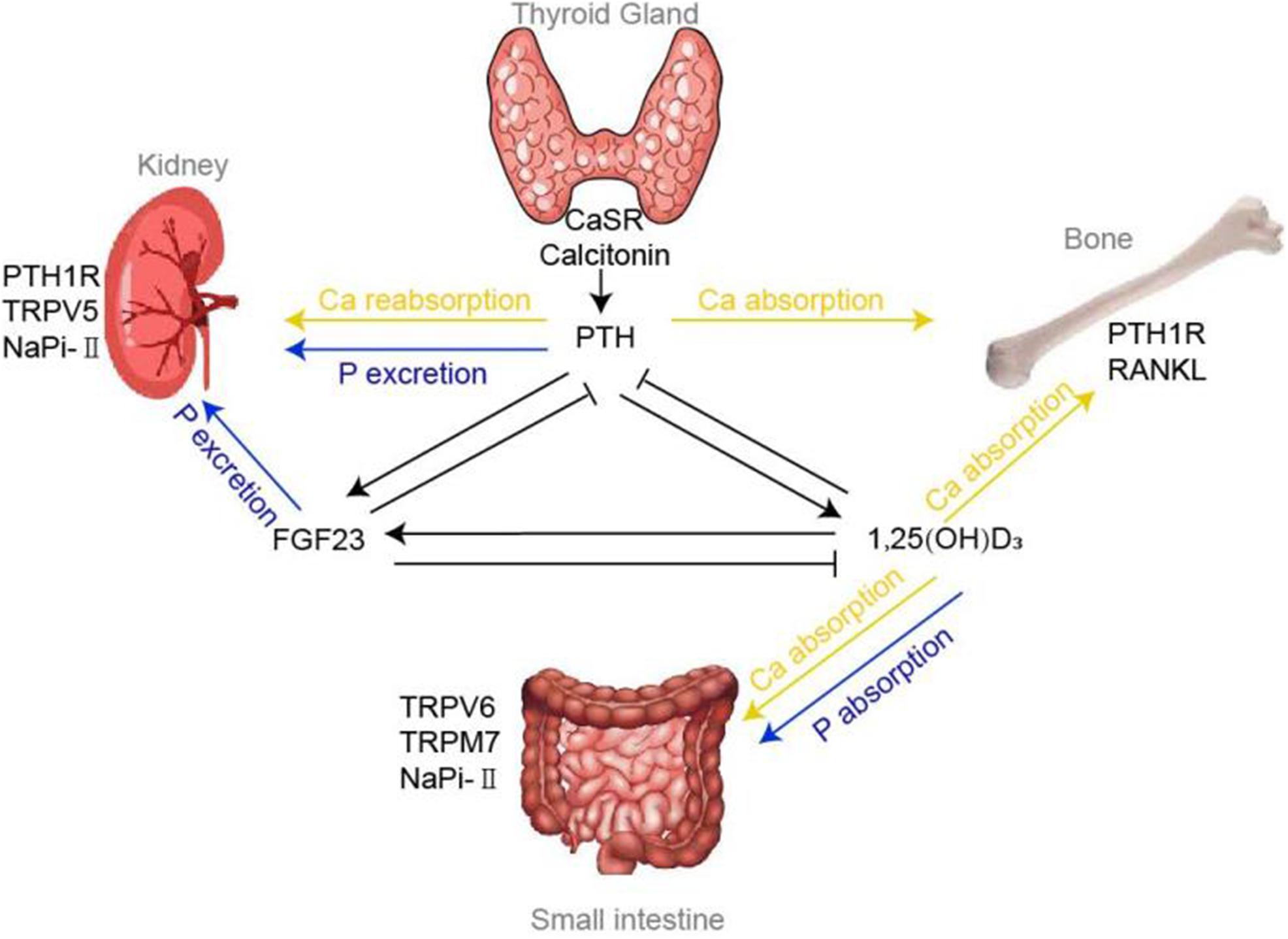
Three significant chemical molecules that affect our planet in many respects are carbonate, calcium carbonate, and calcium phosphoricum. They participate in everything from building rocks to supporting our health maintenance. Knowing these molecules clarifies their use in business, health, and nature.
Carbonate: A Key Player in Nature and Industry
When carbonic acid combines with a base, a chemical known as carbonate results—the carbonate ion (CO₃⁻). Several minerals, including calcite, aragonite, and dolomite, mostly consist of this ion. These minerals are fundamental for the formation of frequently used building materials such as limestone and marble, which are sedimentary rocks. Everything from the basis of buildings to the roads we drive on contains limestone.
Still, the value of carbonate transcends building to include Globally; the carbon cycle depends much on the creation of carbonate rocks. Crucially for preserving the temperature of the Earth, carbon dioxide (CO₂) from the atmosphere is caught in these rocks and serves to control CO₂ levels in the air.
Carbonate is crucial for maintaining our bodies in equilibrium in the biological sphere. In our blood and other biological fluids, the carbonate ion helps to preserve their pH level. This is essential, as many body functions rely on a constant pH. Particularly for creatures like shellfish that utilize calcium carbonate to create their shells, carbonate is vital in the seas in balancing seawater pH, which is essential for the survival of marine life.
Environmental uses for carbonate abound as well. Neutralizing acidic soils and waterways is crucial for restoring natural habitats and helping animals. Lime, a kind of calcium carbonate, for instance, may help acidic lakes and rivers return to a normal pH level, sustaining aquatic life there. Carbonate also helps lower air pollution in manufacturing. For example, power plants employ calcium carbonate to eliminate sulfur dioxide from emissions, lessening the effect of acid rain on the surroundings.
Calcium carbonate: a flexible chemical used in industry and health
Among the most often occurring and adaptable calcium compounds found in nature is calcium carbonate. A central component of shells, coral, and pearls, it is also found in chalk, limestone, and marble. This chemical is very important as it finds many applications in industry and health.
Calcium carbonate is vital in health to preserve robust teeth and bones. One of the primary sources of calcium, a mineral our systems require to maintain strong bones, is Often advised as a dietary supplement to either prevent or cure calcium deficiency—which may lead to disorders like rickets (a disease affecting bone growth in children) or osteoporosis—a condition weakening bones—calcium carbonate is Regular calcium carbonate intake helps elderly persons preserve bone density and lower their fracture risk.
Another aid for digestive health is calcium carbonate. Many antacid medications include it as it rapidly neutralizes stomach acid. This offers relief from acid reflux disease (GERD), heartburn, and other symptoms, including dyspnea. Calcium carbonate lowers stomach acid to minimize pain and possible esophageal injury.
Since calcium carbonate needs stomach acid for maximum absorption, it is most effective with meals. This is a sensible choice for daily supplements as long as they are taken according to the advised instructions to prevent adverse effects like kidney stones or constipation.
Many industrial construction materials have calcium carbonate as their primary component. Building construction depends on it; concrete, mortar, and cement are made with it. Lime is also produced from calcium carbonate; this is crucial for purifying water, stabilizing soils, and manufacturing steel and glass. Its cheap cost and availability make it a basic need in the building sector worldwide.
Beyond building, paper, plastics, and rubber are produced using calcium carbonate mainly as a filler ingredient. Using more reasonably priced raw materials helps reduce production costs. It also makes the paper more brilliant and opaque and enhances the quality of plastic products. Flue gas desulfurization is one environmental preservation project using calcium carbonate. It helps diminish acid rain, transparent sulfur dioxide from industrial emissions, and lessen its adverse effects on ecosystems.

Calcium phosphoricum: Crucially Important for Bone Development and Beyond
Calcium phosphoricum, often called calcium phosphate, is another essential calcium molecule in nutrition and medicine. It consists of two essential elements, calcium and phosphorus, which help to create and preserve strong teeth; furthermore, bone apatite, the mineral most found in our teeth and bones, depends mostly on calcium phosphoricum.
Preserving bone density and tooth strength recommends avoiding dental cavities, gum disease, and osteoporosis. Calcium phosphoricum may encourage bone formation and repair, so it is beneficial in dentistry and medical applications, including bone transplants and dental implants. Its natural fit with our bone tissue makes it perfect for helping to stimulate bone regeneration after surgery or a fracture.
Besides its use in medicine, calcium phosphoricum is vital for nutrition. Many dairy products include it, and it is also a dietary supplement. Those with lactose sensitivity or dietary restrictions limiting their use of dairy products or those who may not receive enough calcium and phosphate from their diet should specifically pay great attention to this.
Appropriate bone formation depends on appropriate calcium phosphoricum throughout growth, including childhood and adolescence. It guarantees that bones develop robustly and healthy, lowering the chance of developing bone problems and fractures later in life.
Calcium phosphoricum is used in homeopathy and conventional medicine to treat various disorders, including general tiredness, dental difficulties, and bone weakening. It is recommended for those recuperating from disease or injury as it enhances general vigor and promotes the body’s healing mechanisms.
Conclusion
Carbonate, calcium carbonate, and calcium phosphoricum are indispensable molecules in many spheres. From their natural existence in rocks and minerals to their vital functions in human health, these molecules are profoundly entwined with our surroundings and everyday life. Carbonate produces Essential sedimentary rocks in part, which is crucial for preserving environmental and biological equilibrium. While calcium phosphoricum is essential for bone strength, dental health, and even sickness recovery, calcium carbonate is the pillar for bone health, digestive help, and industrial uses. Knowing and using these drugs improves our general condition and helps attempts at environmental conservation and sustainable development. Their significance is impossible to overestimate as they still profoundly and subtly change the surroundings.